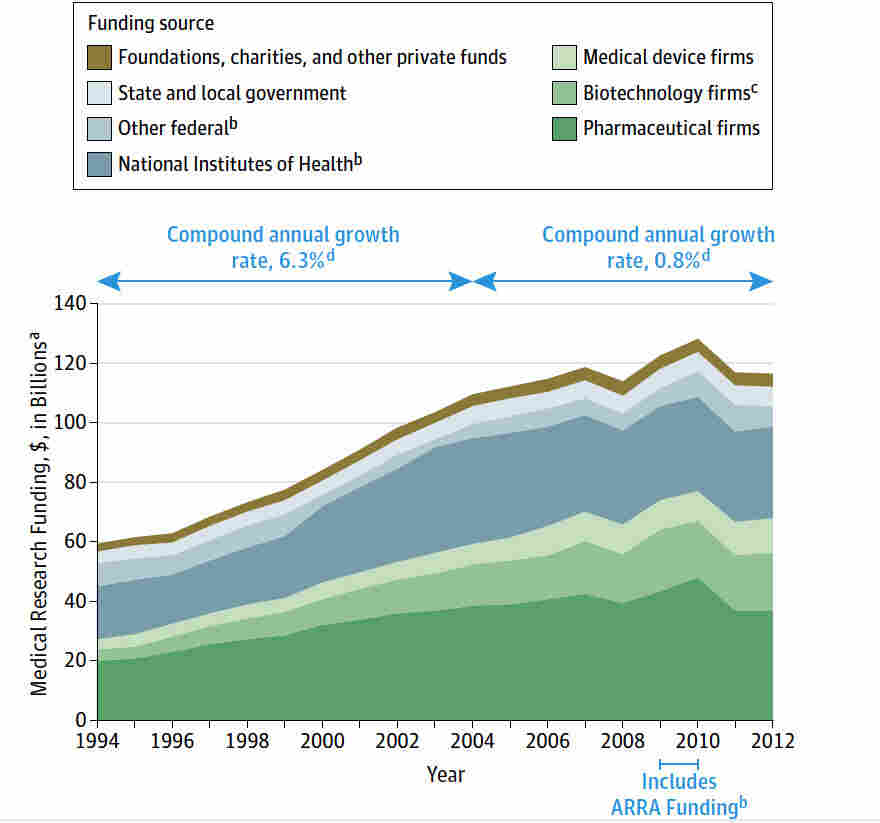
Medical research funding is crucial for advancing health innovations and ensuring patient safety in clinical trials. However, recent funding cuts have raised significant concerns within the research community, particularly in relation to National Institutes of Health (NIH) research grants. The lack of adequate financial resources hampers the operations of Institutional Review Boards (IRBs), which are essential for overseeing research protocols and safeguarding the welfare of human participants. Without sufficient funding, the oversight mechanisms that protect volunteers in studies could weaken, potentially leading to adverse effects on patient safety. The ramifications of these research funding cuts could jeopardize the positive outcomes that medical research, such as those conducted under Harvard’s guidance, aims to achieve.
The financial support for clinical studies, sometimes referred to as biomedical research backing or health investigation grants, plays a fundamental role in the advancement of medical science. As financial resources dwindle, the ability for research institutions to monitor and oversee studies effectively diminishes, raising serious concerns about ethical compliance and participant protection. Alternative funding channels are under scrutiny, particularly as federal research grants face cuts that could disrupt essential oversight functions provided by Institutional Review Boards (IRBs). The effects of this funding reduction ripple through the research community, affecting not just the researchers but also the volunteers who contribute to vital health advancements. The challenge is to maintain robust oversight and ethical standards in light of diminishing research dollars.
The Critical Role of Medical Research Funding in Patient Safety
Medical research funding is fundamental to advancing healthcare and ensuring patient safety. The National Institutes of Health (NIH) provides crucial grants that facilitate innovative studies, which include comprehensive assessments of patient welfare and safety. Without adequate funding, institutions like Harvard are unable to maintain compliance with institutional review boards (IRBs) that safeguard the rights of participants. The recent halt in funding has led to a disruption in this oversight, creating potential risks for patients involved in ongoing and new medical trials.
Moreover, research funding supports essential training programs for researchers and IRB members, enabling them to navigate the complexities of human subject research. This comprehensive funding structure ensures that ethical standards are maintained, minimizing risks while promoting more effective patient-centered outcomes. As federal funding shrinks, the integrity and efficiency of these processes come into question, ultimately affecting the safety measures in place for patients who volunteer for research.
Impact of Research Funding Cuts on Patient Trust
Cuts in research funding significantly impact public trust in medical research, particularly when past scandals have marred the history of such endeavors. Patients who participate in clinical trials rely on the belief that their safety is prioritized, but funding disruptions raise concerns about the thoroughness and ethical standards of IRB reviews. When studies are halted, as seen with Harvard’s SMART IRB, patient participation may decline as skepticism grows regarding the commitment of researchers to uphold safety regulations.
Restoring public confidence in research necessitates a clear demonstration of the value of funding for patient safety. Institutions must address the implications of funding cuts transparently and actively involve community members in discussions about the importance of ethical oversight. Only by doing so can the medical research community hope to rebuild trust, reassuring patients that their well-being is paramount to ongoing research efforts.
IRB Oversight: An Essential Safety Mechanism in Clinical Trials
The role of Institutional Review Boards (IRBs) in safeguarding patient safety cannot be overstated. These boards assess research proposals to ensure that the rights, welfare, and privacy of participants are protected throughout the study’s lifecycle. With the implementation of new NIH policies mandating single IRB review for multisite studies, the expectation for streamlined oversight becomes more critical than ever. Cuts to research funding inhibit these vital processes, jeopardizing the ethical oversight essential for participant safety.
Additionally, IRBs act as a check against potential harm, assessing risk versus benefit in research designs involving human subjects. An influx of funding allows for thorough review processes, training for IRB members, and robust engagement with researchers to instill a culture of safety and ethical responsibility. Without sufficient resources, the capability of IRBs to conduct rigorous evaluations diminishes, leaving patients vulnerable and the integrity of the research process at risk.
The Consequences of Research Funding Cuts on Collaboration
The current landscape of medical research is shaped not only by the innovative work of institutions but also by collaborative efforts across multiple sites. The SMART IRB model exemplifies how shared oversight can facilitate groundbreaking studies. However, recent funding cuts have hindered the ability to forge new collaborations, stalling progress on promising research areas, such as Alzheimer’s disease therapies. Researchers are finding themselves restricted by the bureaucracy brought on by a lack of financial support for essential infrastructure.
Moreover, as various institutions face funding shortages, the opportunity to innovate diminishes significantly. Each halted study not only affects the immediate research goals but also represents a setback in the potential benefits to patients. With rigorous collaboration being essential for comprehensive drug and treatment testing, ineffective funding can stifle advancements that are crucial for patient health outcomes.
Preserving Patient Rights Amidst Funding Challenges
The concept of informed consent is foundational to ethical research practices, ensuring that patients are fully aware of the implications of their participation. However, financial constraints threaten these practices as institutions divert their resources to mitigate the impacts of funding cuts. This can lead to rushed processes that compromise the clarity and honesty required in obtaining informed consent, potentially placing patients at risk.
Furthermore, when funding is limited, educational outreach to participants and communities suffers, making it difficult to engage and prepare prospective subjects adequately. Protecting the autonomy and rights of patients hinges on strong advocacy and support from funded research organizations, which are increasingly strained under the pressures of budget reductions. Addressing these concerns and re-establishing proper funding mechanisms will be essential in safeguarding patient rights in medical research.
Leveraging NIH Research Grants for Patient Protection
NIH research grants play a pivotal role in underpinning projects that prioritize patient protection and safety. These grants facilitate the establishment of comprehensive protocols for studies involving human subjects, ensuring that ethical standards are met, and risks are managed. Funding from the NIH is not merely financial support; it reflects a commitment to uphold the dignity and rights of individuals involved in medical research.
The recent reviews and adaptations in NIH policies, especially regarding IRB oversight, underscore the necessity for consistent funding. These regulations are designed to enhance patient safety by promoting rigorous review processes. Maintaining a steady stream of funding allows for continual adaptation and improvement of these safety protocols, fostering a more reliable research environment that prioritizes patient well-being.
Community Engagement in Medical Research Amidst Funding Cuts
Community involvement is essential in shaping the landscape of medical research, particularly in times of funding cuts. Engaging participants in the research process not only enhances transparency but also fosters a sense of trust and ownership over the research outcomes. Initiatives that place patients and communities at the forefront of research agendas are crucial for addressing their needs and concerns, especially when adverse funding decisions threaten to limit collaborative opportunities.
As academic and clinical research institutions work to navigate budget constraints, it is essential to maintain robust communication channels with community stakeholders. By listening to the voices of those impacted by research, including patient representatives, institutions can adapt their funding strategies to align with community needs, ultimately supporting better patient safety practices amidst challenging financial climates.
Historical Context and Its Implications for Modern Research
Historical injustices in medical research serve as a somber reminder of the ethical dilemmas that necessitate the safeguards we have today, including IRBs and stringent funding regulations. Events such as the Tuskegee syphilis study remain etched in public consciousness, driving home the significance of transparency and ethical oversight in medical research. Understanding this historical context is crucial for recognizing the importance of maintaining strong funding mechanisms that support ethical research practices.
The evolution of research ethics has led to robust structures designed to protect participants, but these frameworks require sustained investment to thrive. Funding cuts not only undermine current research efforts but can also open the door to past mistakes being repeated. Reinforcing the lessons learned from history will enable the medical community to adopt cautious, empathetic approaches that prioritize participant protection and trust, critical elements that underpin the success of future research.
Future Directions in Medical Research Funding and Patient Safety
Looking ahead, the medical research community faces the challenge of securing sustainable funding to ensure patient safety remains a fundamental priority. Innovative funding models that embrace public-private partnerships could emerge as a solution to the current limitations posed by federal cuts. By engaging diverse stakeholders, researchers can develop comprehensive strategies that encompass not just scientific inquiry but also community impact and safety measures.
Moreover, advocating for a reevaluation of funding priorities at national levels could lead to the reinstatement of essential financial support for research that prioritizes patient safety. Encouraging policymakers to recognize the long-term benefits of investing in research infrastructure will play a crucial role in shaping the future landscape of medical research, ultimately benefiting patients and communities alike.
Frequently Asked Questions
What impact do research funding cuts have on patient safety in medical research?
Research funding cuts significantly undermine efforts to ensure patient safety in medical research. For example, funding from organizations like the NIH is crucial for maintaining the Institutional Review Board (IRB) oversight, which protects the rights and welfare of human research participants. Without adequate funding, many studies face operational disruptions, halting recruitment and threatening the integrity of existing research efforts.
How does NIH research funding affect the oversight of medical studies?
NIH research funding is essential for the review and ongoing oversight of medical studies conducted on human participants. This funding supports the IRB processes that are legally required to ensure compliance with ethical standards. The suspension of NIH grants can consequently impede the oversight functions critical to safeguarding patient participants, which may lead to compromised research integrity.
What role does SMART IRB play in the context of medical research funding?
SMART IRB (Single Institutional Review Board) facilitates the ethical oversight of multi-site medical research funded by entities like NIH. It streamlines the IRB process, allowing for quicker approval of studies across multiple institutions. However, recent funding cuts disrupt this system, creating challenges in ensuring patient safety and delaying critical medical research initiatives.
Why are IRB reviews important in the landscape of medical research funding and patient safety?
IRB reviews are vital because they ensure that all aspects of medical research comply with ethical standards designed to protect patient safety. This oversight involves evaluating study designs, consent processes, and risk assessments. When research funding is cut, IRBs may struggle to operate effectively, which can result in inadequate protection for participants involved in medical studies.
How do federal grants like those from NIH influence the dynamics of collaboration in medical research?
Federal grants, such as those from NIH, are pivotal in promoting collaboration among research institutions. They fund initiatives like the SMART IRB, which simplifies multi-site studies. A halt in these grants can stifle collaborative efforts, leading to delays and complications in important medical research that relies on shared resources and expertise.
What historical context influences current practices in medical research funding and patient safety?
Historical events, such as the Tuskegee Syphilis Study, have profoundly shaped current practices in medical research funding and the emphasis on patient safety. These incidents underscored the necessity for robust ethical oversight and enforcement mechanisms, primarily provided by IRBs. Funding for these oversight functions ensures that lessons from the past continue to inform and improve the safety and rights of research participants today.
| Key Points |
|---|
| The Trump administration’s funding freeze of over $2 billion affects medical research and patient safety. |
| A stop-work order was issued for the SMART IRB contract, disrupting oversight of multi-site studies. |
| The SMART IRB facilitates collaborative oversight among hospitals, universities, and federal agencies. |
| IRBs ensure compliance with ethical standards in human research and protect participants’ rights. |
| NIH funds support necessary reviews, approvals, and oversight, which are crucial to participant safety. |
| Funding cuts can lead to halted research, increased harms to participants, and public skepticism towards medical studies. |
| Historical misdeeds in research highlight the importance of oversight and ethical conduct in studies today. |
| Continued funding and institutional support are vital for maintaining patient safety and advancing research. |
Summary
Medical research funding is critical for maintaining the safety and rights of patients involved in studies. The recent freeze in federal funding disrupts established oversight systems like SMART IRB, leading to concerns over the well-being of research participants. The implications of these funding cuts underscore the necessity of continued investment in medical research, particularly to uphold ethical standards and public trust.