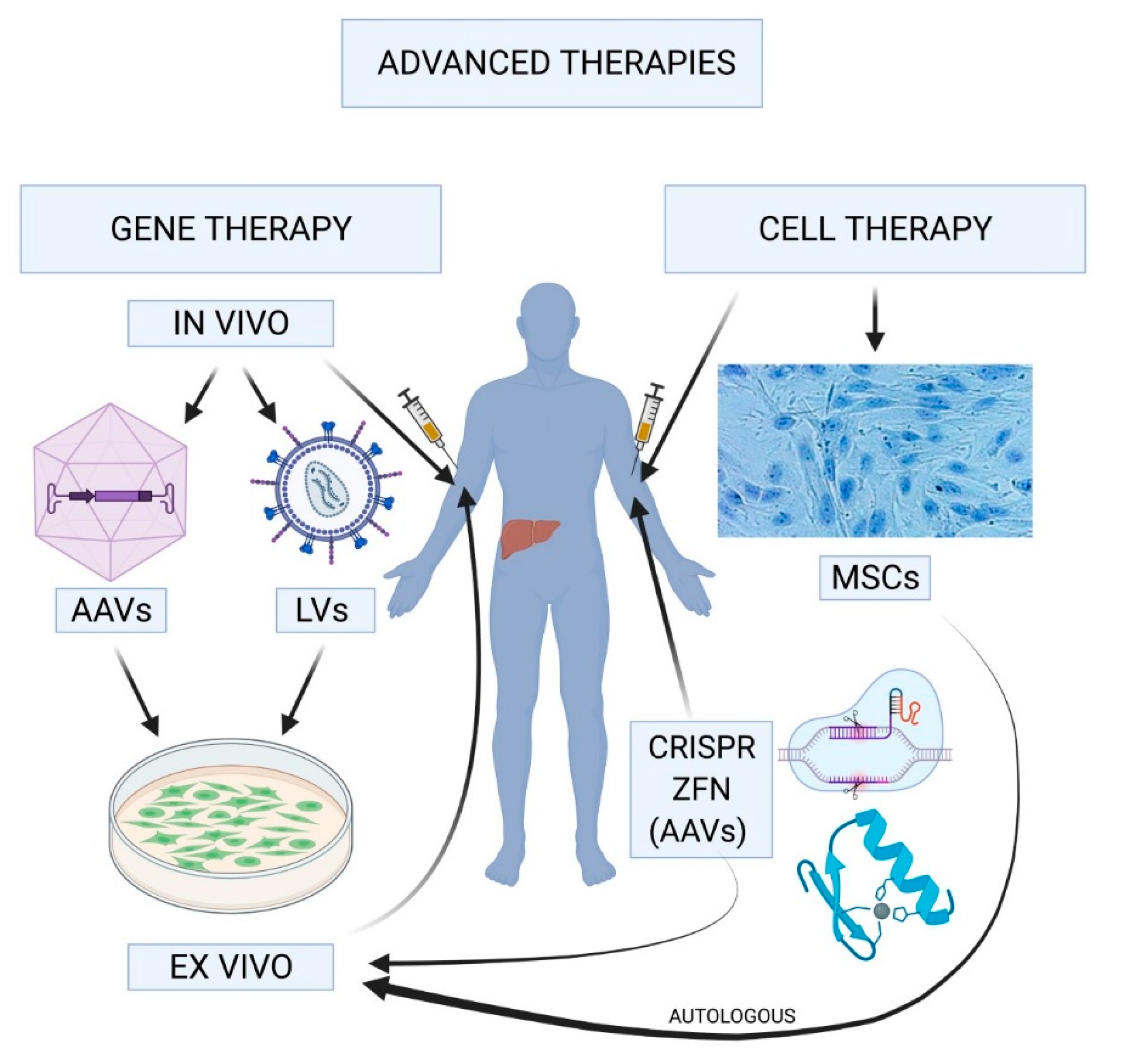
Gene therapy for hemophilia represents a groundbreaking advancement in hemophilia treatment, holding the potential to transform the lives of those affected by this genetic disorder. With the recent FDA approval of Hemgenix, a novel gene therapy, patients like Terence Blue are experiencing remarkable improvements in their condition, reducing reliance on traditional clotting factor treatments. The benefits of gene therapy extend beyond mere symptom management; they offer a path towards a future where frequent infusions and constant worry over bleeding can become a thing of the past. These advancements in hemophilia B treatment signify a significant leap forward, bringing hope to thousands and highlighting the importance of ongoing research in genetic medicine. As the field continues to evolve, understanding the implications of these therapies becomes crucial for both patients and healthcare providers alike.
When discussing innovative treatments for hemophilia, terms such as genetic intervention and cellular therapy often come into play, encapsulating the essence of modern medical breakthroughs. These methods aim to address the underlying causes of hemophilia, moving away from conventional approaches that rely heavily on clotting factor replacements. The emergence of therapies like Hemgenix signifies a shift in hemophilia care, providing hope for effective, long-lasting solutions. This pivot towards utilizing genetic modifications underscores the continuous evolution of hemophilia management strategies, revealing the potential for improved patient outcomes and enhanced quality of life. By embracing these innovative treatments, we open the door to a new era in treating genetic disorders.
The Rise of Gene Therapy for Hemophilia B
Gene therapy is revolutionizing the treatment landscape for various medical conditions, and hemophilia is no exception. Hemophilia B, a genetic disorder that leads to deficiency of clotting factor IX, has seen significant advancements in treatment options. The introduction of Hemgenix, the first FDA-approved gene therapy for hemophilia B, marks a transformative milestone for patients who have relied on traditional infusions of clotting factors. With promising clinical trial results showcasing that many treated patients do not require regular factor IX prophylaxis anymore, gene therapy offers hope for a long-term solution.
The underlying technology of Hemgenix allows for a single administration to potentially correct the genetic defect causing hemophilia. This addresses a long-standing challenge in hemophilia management: the need for frequent infusions of clotting factors, which can be both inconvenient and costly. As patients experience the benefits of gene therapy, the medical community is watching closely to assess not only clinical outcomes but also the long-term implications of such therapies on their quality of life.
Frequently Asked Questions
What are the benefits of gene therapy for hemophilia treatment advancements?
Gene therapy for hemophilia offers several important benefits including a significant reduction in the need for frequent injections of clotting factors, enhanced quality of life by mitigating the emotional and physical burden of the disease, and the potential to provide long-lasting effects that can change the management of hemophilia permanently.
How does Hemgenix gene therapy improve outcomes for patients with hemophilia B updates?
Hemgenix gene therapy specifically targets hemophilia B by delivering a corrected version of the gene responsible for producing clotting factor IX into the liver, leading to increased production of this clotting factor and significantly reducing bleeding episodes. Early data indicates that many patients maintain stable factor levels without regular infusions post-treatment.
What challenges do patients face in accepting new gene therapy benefits for hemophilia treatments?
Patients may face challenges such as high treatment costs, insurance coverage uncertainties, awareness about new therapies, and the hesitation to shift from traditional clotting factor treatments to gene therapy, influenced by social factors and personal experiences with their condition.
What is the significance of Hemgenix gene therapy in the context of hemophilia treatment advancements?
Hemgenix represents a transformative advancement in hemophilia treatment by offering a potential one-time therapy that can reduce or eliminate the need for ongoing regular treatments, thus providing patients with greater autonomy and reduced fear of bleeding complications linked to hemophilia.
How does gene therapy for hemophilia compare to traditional clotting factor treatments?
Unlike traditional clotting factor treatments that require regular injections, gene therapy for hemophilia aims to correct the underlying genetic defect, potentially allowing patients to produce their own clotting factors, thus significantly reducing the frequency of administration and associated burdens.
Can gene therapy for hemophilia B replace conventional treatments entirely?
While gene therapy for hemophilia B, like Hemgenix, has shown promising results in trials, it may not completely replace conventional treatments for everyone. Some patients may still need episodic treatment, and ongoing monitoring and adjustments may be required.
What future developments can be expected in gene therapy for hemophilia treatments?
Future developments in gene therapy for hemophilia may include improved delivery mechanisms, reduced costs, expanded eligibility for various types of hemophilia, and possibly combination therapies that enhance effectiveness and patient outcomes.
What role does patient acceptance play in the success of gene therapy for hemophilia?
Patient acceptance is crucial for the success of gene therapy for hemophilia as it influences treatment adherence, engagement with healthcare providers, and the overall adoption of innovative therapies that may offer better long-term management of the disease.
Are there any specific risks associated with Hemgenix gene therapy for hemophilia B?
Like any medical treatment, Hemgenix does carry risks such as immune reactions, liver enzyme elevation, and potential for long-term unknown effects. Thus, patients are typically monitored closely following therapy.
How can hemophilia treatment advancements through gene therapy impact the lives of patients?
Advancements in hemophilia treatment through gene therapy can greatly enhance the quality of life for patients by reducing the fear of bleeding, eliminating burdensome treatment schedules, and allowing for more freedom in daily activities.
| Key Points |
|---|
| Terence Blue, the first patient in New England to receive gene therapy for hemophilia B at Brigham and Women’s Hospital. |
| The therapy, called Hemgenix, was FDA approved in November 2022 and aims to stop the need for frequent clotting factor injections. |
| While gene therapy holds transformative potential, challenges remain regarding costs and patient acceptance in the market. |
| Blue’s story highlights the long-term management of hemophilia and the recent advancements in treatment options. |
| With gene therapy, hemophilia B patients like Blue may significantly reduce dependency on traditional treatments and needles. |
Summary
Gene therapy for hemophilia has emerged as a groundbreaking treatment option, promising to transform the lives of patients like Terence Blue who have spent years managing their condition. The recent FDA-approved gene therapy, Hemgenix, represents a significant leap towards reducing the need for frequent injections of clotting factors, offering hope for a better quality of life. While the market faces challenges including high treatment costs and patient acceptance, the potential for gene therapy to provide lasting effects without the burden of daily needles is becoming increasingly tangible. As research progresses and more patients receive such innovative treatments, optimism grows for the future of hemophilia management.