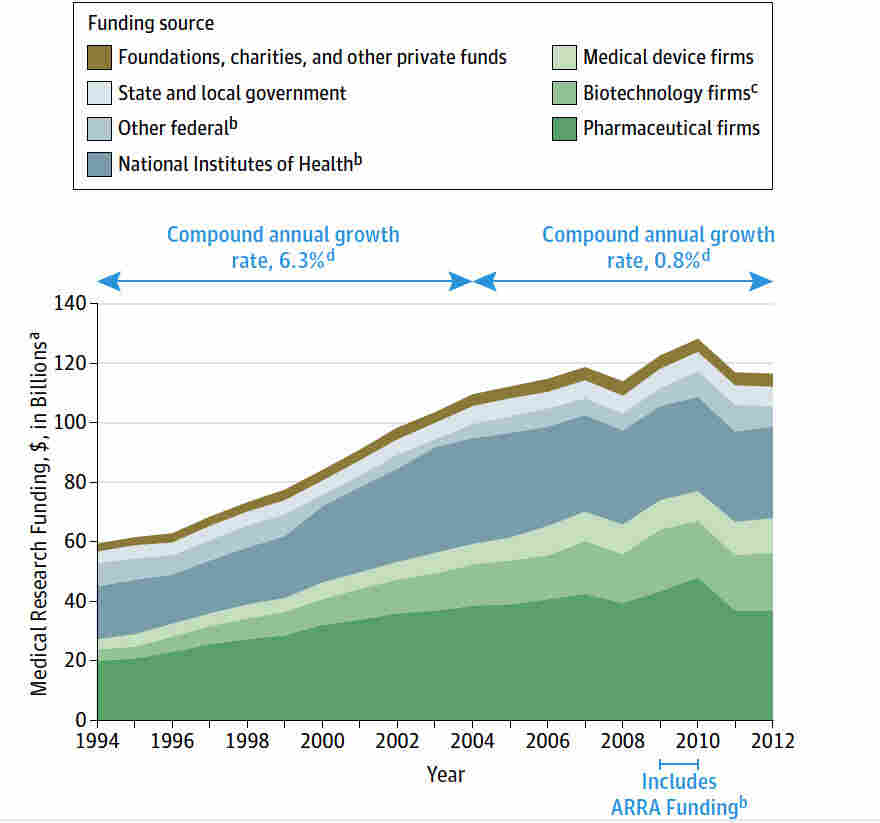
Medical research funding is vital for advancing scientific understanding and ensuring patient safety in clinical studies. Without adequate financial support, the impact of funding on research becomes detrimental, disrupting crucial oversight mechanisms that protect participants. Institutions like Harvard have faced significant challenges due to a freeze in federal grants, which highlights the essential role of funding in facilitating Institutional Review Board (IRB) functions. These boards are responsible for safeguarding patient protection in research, ensuring that studies adhere to ethical practices and regulations. As a result, the effects of NIH funding and its consequences on the safety and effectiveness of medical investigations cannot be overstated.
Financial support for healthcare research, often referred to as medical research financing, plays a pivotal role in shaping the landscape of clinical trials. When there is a lack of investment, the intricacies involved in maintaining participant safety and ethical oversight can be severely compromised. Resources from funding agencies, such as the NIH, underscore their importance and effectiveness in upholding participant welfare and ensuring rigorous review processes through IRB oversight. Moreover, the ramifications of halted funding ripple through the research community, potentially eroding public trust and hindering progress in medical innovations that benefit countless patients. In essence, the availability of research funding is deeply intertwined with the integrity and success of scientific endeavors in the health sector.
The Importance of Medical Research Funding in Patient Safety
Medical research funding is crucial for advancing healthcare and improving patient safety. Funding from organizations like the National Institutes of Health (NIH) ensures that necessary resources are available for conducting thorough clinical studies. These studies, thoroughly vetted by Institutional Review Boards (IRBs), investigate the potential benefits and risks associated with new therapies, medications, and medical devices. When funding is stable, research initiatives can move forward efficiently, allowing for the continued evaluation of patient protection measures and safety protocols within the medical community.
However, disruptions in funding, such as the recent halt of $2 billion in federal research grants to institutions like Harvard, pose significant risks to patient safety. Not only do these funding cuts stall ongoing studies, but they also hinder the implementation of crucial safety measures that protect participants. The lack of resources can lead to delays in approving necessary trials or modifications to existing studies, ultimately affecting how effectively researchers can address potential adverse effects.
Impact of Funding Cancellations on Research and Ethics
Cancellations of medical research funding present a serious challenge to the ethical standards set by IRBs, which exist to protect the rights and welfare of research participants. When funding is removed, the comprehensive review processes that IRBs provide may struggle to maintain their thoroughness, leading to potential lapses in safety oversight. Research professionals rely on this funding to adhere to ethical practices; without it, both the integrity of research and the security of participants may be compromised.
Furthermore, the cancellation of funding can lead to a systemic breakdown in the collaborative networks that IRBs rely on to ensure comprehensive oversight across multiple sites. With the halt of funds, new institutions cannot join ongoing trials, which stifles innovation and the sharing of vital knowledge among researchers. The resulting fragmentation can heighten ethical concerns related to informed consent and patient safety while breeding mistrust within the communities that researchers aim to serve.
The Role of IRBs in Safeguarding Participants
Institutional Review Boards (IRBs) play a vital role in safeguarding research participants by ensuring that ethical standards are met throughout the lifecycle of medical studies. These boards are responsible for reviewing recruitment strategies, monitoring potential risks, and ensuring that informed consent processes are transparent and effective. The knowledge and oversight provided by IRBs become even more critical in the context of tight funding, where the potential for compromising participant welfare may increase without adequate fiscal resources.
Additionally, IRBs serve as educators for researchers, instilling best practices in protecting participants. By emphasizing ethical conduct and oversight, they foster an environment where patient protection is paramount, thereby reinforcing public trust in the research process. In light of funding challenges, IRBs must continue to advocate for stringent ethical standards to maintain the integrity of medical research and the safety of participants.
Evaluating the Historical Context of Medical Research Funding
To understand the pivotal role of funding in medical research, it is essential to consider historical events that have influenced current ethical guidelines. Historical abuses, such as the Tuskegee Syphilis Study and the inhumane treatment of participants at Willowbrook State School, have shaped the stringent requirements set forth by IRBs to ensure participant protection. These events underscore the necessity for well-funded oversight processes that monitor and facilitate ethical research practices.
The lessons learned from history emphasize the correlation between funding availability and protective measures for patients. A well-funded research environment enables institutions to implement comprehensive ethical frameworks and oversight mechanisms that have emerged since these dark chapters in medical history. As funding becomes increasingly variable, it remains vital that institutions prioritize the ethical review of research practices to safeguard the public’s trust and welfare.
Consequences of Research Funding Cuts on Innovation
Research funding cuts pose severe consequences for innovation in medical science and therapeutic development. A halt in financial support can mean that institutions and researchers are unable to explore promising avenues for treatment, particularly in urgent areas like Alzheimer’s disease or cancer therapies. With significant federal funding, researchers can tackle complex problems collaboratively, share insights, and accelerate the development of life-saving treatments. Unfortunately, without adequate funding, these crucial opportunities for innovation become significantly limited.
Moreover, the interplay between funding and collaborative initiatives is critical for advancing medical research. Systems like the SMART IRB, designed to streamline multi-site studies, heavily depend on consistent funding to operate effectively. The potential to support groundbreaking treatments diminishes when funding is cut, leading to increased delays in bringing new therapies to market. The broader implications of these cuts not only affect researchers but ultimately impact patient access to emerging medical advancements.
Protecting Patient Rights: The Financial Aspect
Patient rights and safety are intrinsically linked to the financial resources allocated for medical research. Funding from governmental and private entities is vital in upholding the standards that protect participants during clinical trials. Without adequate financial backing, the ability of IRBs and research institutions to monitor compliance with ethical guidelines may be significantly undermined, leading to increased risks for participants and diminished trust in the research process.
Furthermore, when financial resources are limited, institutions may struggle to implement best practices in terms of participant communication and education about their rights. Robust funding is necessary to support initiatives that inform participants of their rights and the potential risks and benefits involved in research. As a result, ensuring adequate funding for medical research is not merely a financial issue—it is fundamentally about safeguarding the dignity and welfare of those participating in clinical studies.
NIH Funding Effects on Collaborative Research
The effects of NIH funding on collaborative research initiatives cannot be overstated. NIH grants are often instrumental in driving forward multi-site studies that require coordinated oversight and resources across various institutions. This funding fosters an environment of collaboration, allowing researchers to share data, resources, and expertise effectively. Such collaboration is crucial in medical research, as it accelerates the pace at which new knowledge can be generated and implemented for patient care.
However, when NIH funding is lost or suspended, the collaborative spirit of research can suffer. Institutions may become isolated in their studies, leading to redundancies and inefficiencies that can hinder progress. The resulting fragmentation not only impairs the ability to conduct thorough investigations but can also influence how findings are disseminated and utilized in clinical practice, ultimately affecting patient care and treatment outcomes.
The Ethical Imperative of Funding in Medical Studies
Funding in medical studies is not just a bureaucratic necessity; it is an ethical imperative. Adequate funding ensures that studies adhere to rigorous ethical standards, thereby protecting participants and enhancing research quality. When researchers are assured of stable financial support, they can focus on designing robust studies with comprehensive safety measures, which directly contributes to the well-being of participants. IRBs play a crucial role in this system by evaluating the proposed research against ethical benchmarks, helping to ensure that patients are treated with respect and dignity.
Moreover, when funding is secure, researchers invest in the training and education required for all individuals involved in a study, fostering a culture of safety and ethical responsibility within research teams. This investment is essential for maintaining a high standard of ethical conduct in medical research, ultimately benefitting the public and advancing medical science. Therefore, reinforcing the importance of funding is indispensable to uphold the ethical foundations of medical research.
Public Trust and Research Funding Stability
Public trust is vital for the success of medical research, and funding stability plays a significant role in fostering this trust. When sufficient resources are allocated for ethical oversight, compliance, and protection of participants’ rights, the public is more likely to have confidence in the research process. Trust is further enhanced when stakeholders can see the tangible benefits of funded research through improved health outcomes and advances in medical practice.
Conversely, disruptions in funding can lead to public skepticism regarding the integrity of research. For instance, recent cuts to funding have raised concerns among potential participants about whether their safety and rights are adequately protected during studies. Therefore, maintaining a steady stream of funding is essential not only for the advancement of medical science but also for preserving the trust of patients and the communities impacted by research.
Frequently Asked Questions
What is the impact of funding on medical research and patient safety?
Funding plays a crucial role in medical research, significantly contributing to patient safety. Well-funded research allows for thorough oversight through Institutional Review Boards (IRBs), ensuring compliance with ethical guidelines. Without sufficient funding, oversight mechanisms may suffer, risking participant safety and research integrity.
How do NIH funding effects impact patient protection in research?
NIH funding affects patient protection by facilitating thorough review and oversight by IRBs. These boards ensure that studies comply with safety regulations, thereby safeguarding participants’ rights and welfare. Adequate NIH support is essential for maintaining robust patient protection in ongoing medical research.
What role do IRB functions play in ensuring safety in medical studies?
IRB functions are critical in ensuring safety in medical studies. They meticulously assess research proposals, evaluate risks, and ensure informed consent processes are in place. This oversight protects patients by preventing ethical breaches and fostering a trustworthy research environment.
How does the halt in funding disrupt the impact of funding on research?
A halt in funding disrupts the impact of funding on research by delaying studies and limiting the resources available for IRB oversight. This may hinder the commitment to ethical practices, ultimately affecting patient safety and community trust in medical research.
What is the relationship between the impact of funding on research and the safety of patients?
The relationship between the impact of funding on research and patient safety is direct; adequate funding ensures comprehensive oversight by IRBs, which is essential for protecting patient rights and welfare during clinical trials. Cutbacks in funding can compromise these protective measures.
| Key Points | Details |
|---|---|
| Impact of Funding Cuts | The Trump administration’s freeze of over $2 billion in federal research grants, including a stop-work order on SMART IRB, disrupts oversight of patient safety in research. |
| Role of IRBs | IRBs ensure the protection of research participants through review processes, informed consent, and oversight mechanisms. |
| Historical Context | Incidents like the Tuskegee study highlight the critical need for ethical oversight in medical research. |
| Consequences for Patients | Delays in studies and collaboration due to funding cuts can compromise participant safety and hinder medical breakthroughs. |
| Importance of Collaboration | SMART IRB was created to streamline collaboration among multiple research sites, enhancing efficiency and safety in medical studies. |
Summary
Medical research funding is crucial for ensuring the safety and rights of patients involved in clinical studies. The recent funding cuts have disrupted essential oversight mechanisms, such as the SMART IRB, leading to significant delays and potential risks to participant welfare. Without adequate funding, research institutions are unable to effectively collaborate, further jeopardizing public trust in the medical research enterprise. Investing in medical research funding is imperative to protect patients and promote ethical standards in research.