Microglial Research: A New Frontier in Alzheimer’s Treatment
Microglial research has become a pivotal area of study in understanding the complex interplay between the brain’s immune system and neurodegenerative diseases such as Alzheimer’s disease. These specialized cells are critical for maintaining brain health, as they monitor for signs of damage and engage in vital processes like synaptic pruning, the removal of unnecessary neuronal connections. However, recent findings indicate that dysregulated microglial activity may exacerbate conditions like Alzheimer’s, leading to detrimental outcomes. Pioneering work by researchers such as Beth Stevens has illuminated these cellular processes, offering hope for new biomarkers and treatment strategies for millions affected by cognitive decline. As science continues to uncover the roles of microglia in disease, we edge closer to innovative interventions that could transform patient care.
Research surrounding glial cells, particularly microglia, stands at the forefront of unraveling mysteries surrounding neurodegenerative disorders. These cells constitute the brain’s immune defense, helping to eliminate damaged neurons and engaging in synaptic remodeling, essential for healthy brain function. Nevertheless, imbalances in microglial activation have been linked to disorders like Alzheimer’s and Huntington’s disease, raising questions about their dual role in health and disease. Investigations led by experts like Beth Stevens are shedding light on these mechanisms, fostering the development of novel diagnostic and therapeutic approaches. By delving deeper into the brain’s supportive cell populations, we may uncover critical insights that facilitate better management of cognitive disorders.
Understanding Microglial Cells in Neurodegenerative Diseases
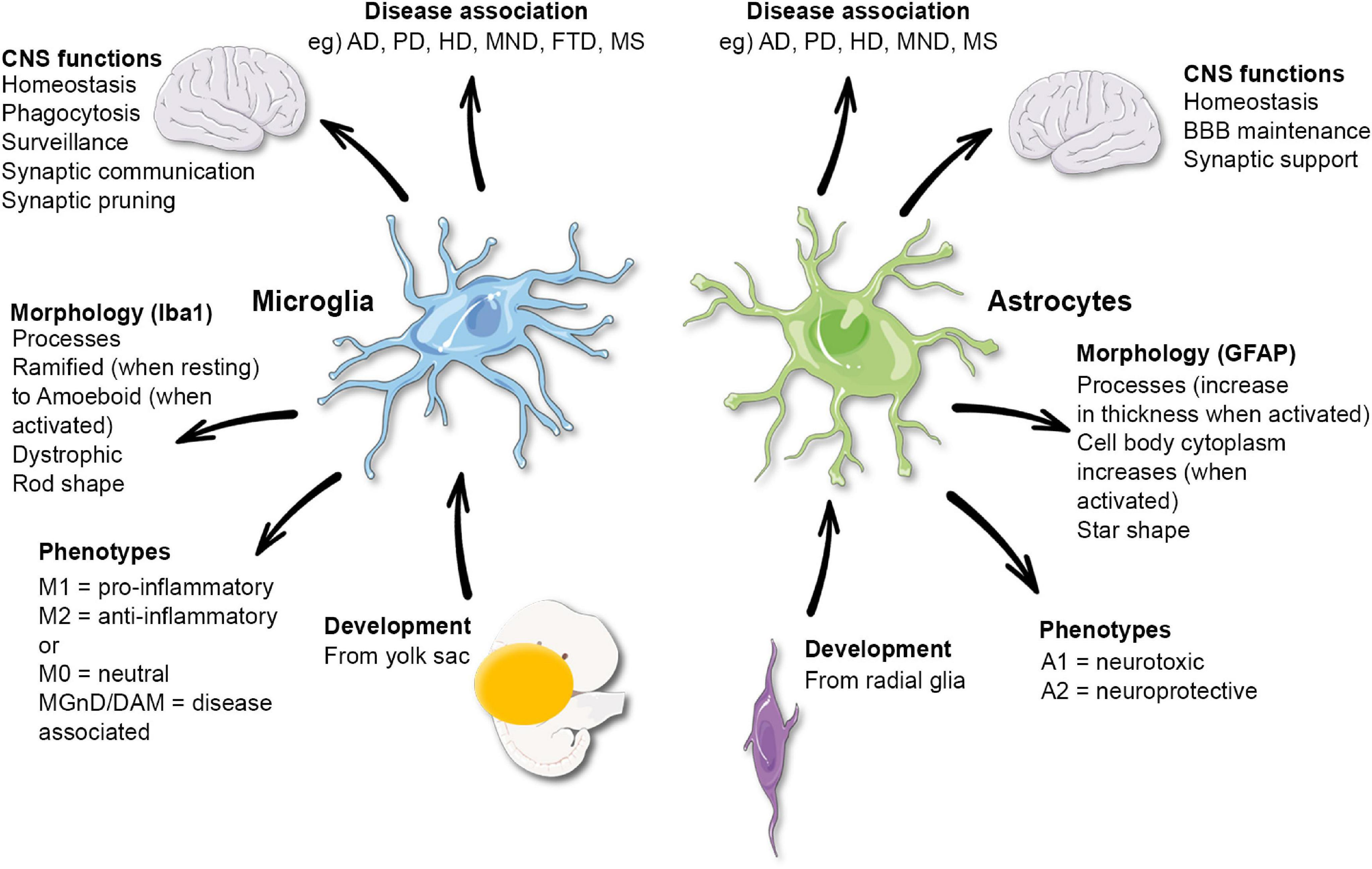
Microglial cells are essential components of the brain’s immune system. These specialized cells constantly survey the brain for pathogens and debris, thereby playing a crucial role in maintaining neuronal health. In recent studies, particularly through the work of Beth Stevens, it has been revealed that microglia not only defend against infections but also engage in synaptic pruning — a process that fine-tunes neuronal connections during brain development. This dual role highlights the significance of microglial research in understanding various neurodegenerative diseases.
However, malfunctioning microglia can negatively impact brain health. When these cells begin to prune synapses excessively, they may contribute to the development of conditions such as Alzheimer’s disease and Huntington’s disease. Stevens’ research has illuminated how improper synaptic pruning by microglia is a pivotal factor in these disorders, leading to the loss of neuronal connections and cognitive decline. Thus, advancing our knowledge about microglial functions and dysfunctions is vital in unraveling the complexities of neurodegenerative diseases.
The Role of Synaptic Pruning in Alzheimer’s Disease
Synaptic pruning is a natural process that refines synaptic connections, ensuring efficient communication between neurons. In healthy brains, this process is crucial for learning and memory. However, in the context of Alzheimer’s disease, abnormal synaptic pruning mediated by microglia has been linked to neural degeneration. Research led by Beth Stevens demonstrates that misregulated pruning can exacerbate Alzheimer’s symptoms, as it may lead to the selective elimination of pivotal neural connections necessary for cognitive function.
Beth Stevens’ investigations into the role of microglial cells in synaptic pruning have opened up new avenues for therapeutic strategies. By developing a better understanding of how microglia behave in disease states, scientists are beginning to identify potential biomarkers for early detection of Alzheimer’s disease and other neurodegenerative conditions. This could have a profound impact on timing and efficacy of interventions, ultimately improving the quality of life for millions of individuals afflicted by these diseases.
Research Breakthroughs: The Impact of Beth Stevens’ Work
Beth Stevens’ groundbreaking research has transformed our comprehension of the brain’s immune system, particularly focusing on microglial functions. Her work has provided clarity on how these cells protect the brain while also uncovering the dark side of their activity in the context of neurodegenerative diseases. Significant findings from her lab have revealed that a disruption in microglial function can lead to worsening conditions in patients with Alzheimer’s disease, illustrating the complex interplay between immunity and neurodegeneration.
With substantial support from NIH and other federal agencies, Stevens has pursued vital research that bridges basic science with clinical applications. This has fostered groundbreaking advancements in identifying new treatment pathways, such as targeting microglial activity to improve synaptic health in neurodegenerative diseases. Her contributions underscore the importance of funding basic research, which lays the foundation for future innovations in combating disorders like Alzheimer’s.
The Future of Neurodegenerative Disease Treatment
As research into microglial cells continues to unveil their crucial role in neurodegenerative diseases, the future of treatment looks promising. The identification of new biomarkers, as highlighted in Beth Stevens’ work, paves the way for more precise and earlier interventions for diseases like Alzheimer’s. This shift from reactive to proactive treatment approaches could drastically alter the prognosis for millions of patients by allowing for timely and targeted therapies.
Moreover, the integration of findings from studies on microglia and synaptic pruning holds potential for developing novel drug therapies aimed at modulating microglial activity. By fine-tuning the balance of immune response in the brain, scientists hope to mitigate neural damage and restore cognitive functions. This holistic approach to understanding and treating neurodegenerative diseases emphasizes the interconnectedness of immune processes and neuronal health, presenting new opportunities for enhancing patient care.
Exploring Neuroinflammation in Alzheimer’s Disease
Neuroinflammation is increasingly recognized as a central player in the progression of Alzheimer’s disease. Microglial cells are not only involved in synaptic pruning but also in the inflammatory response within the brain. When these cells respond to injury or neurodegeneration, they can either promote healing or contribute to chronic inflammation that exacerbates disease progression. Understanding the nuances of this response is crucial for developing effective interventions.
Beth Stevens’ research has provided valuable insights into how neuroinflammation can be managed therapeutically. By investigating the mechanisms that lead to dysfunctional microglial responses, her studies aim to uncover methods to harness the beneficial aspects of microglial activity while dampening the harmful inflammatory processes. This dual approach opens up possibilities for creating treatments that could slow the progression of Alzheimer’s disease and improve outcomes for those affected.
Impact of Federal Funding on Alzheimer’s Research
Federal funding, particularly from organizations like the NIH, plays a vital role in advancing Alzheimer’s research. Prominent researchers like Beth Stevens have repeatedly emphasized how critical funding has been to their groundbreaking studies. It allows for innovative research that not only furthers our understanding of diseases like Alzheimer’s but also catalyzes the development of new therapies and diagnostic tools.
The success of Stevens’ lab showcases the tangible outcomes of such investments. By supporting exploratory research into microglial biology and neurodegeneration, federal funding has facilitated pivotal discoveries that may lead to better strategies for managing Alzheimer’s disease. Continuous investment in this field is essential to sustain progress, enabling scientists to tackle the complex challenges posed by neurodegenerative diseases.
The Genetics of Alzheimer’s Disease and Microglial Research
Recent advances in genetic research have shed light on the hereditary aspects of Alzheimer’s disease. Studies have identified genes that are linked with increased risk for developing this debilitating condition, and many of these genes are implicated in the functioning of microglial cells. By understanding the genetics behind Alzheimer’s, research can better inform targeted therapies that address the underlying causes of disease.
Beth Stevens’ work intersects with genetic research by exploring how specific genetic variations can alter microglial behavior and contribute to disease mechanisms. This intersection of genetics and immunology presents exciting opportunities for the development of gene therapy approaches aimed at normalizing microglial function, ultimately reducing the risk or severity of Alzheimer’s symptoms. Future investigations are likely to deepen our understanding of how genetic predispositions can inform therapeutic strategies.
The Role of Basic Science in Medical Advancements
Basic science research provides the foundational knowledge that drives advancements in medicine. By exploring fundamental questions about biological processes, scientists like Beth Stevens have laid the groundwork for significant breakthroughs in understanding diseases such as Alzheimer’s. This type of research is often supported by federal funding, which is crucial for investigating complex interactions within the brain.
The insights gained from basic science extend beyond theoretical knowledge; they inform clinical approaches that can directly benefit patients. For instance, Stevens’ revelations about microglial function could lead to new diagnostic markers or treatment protocols for Alzheimer’s disease. This highlights how the synergy between basic research and clinical application is vital for transforming scientific discoveries into practical solutions for neurological disorders.
Collaborative Efforts in Alzheimer’s Disease Research
Collaboration among researchers is essential in the battle against Alzheimer’s disease and other neurodegenerative disorders. By pooling expertise from various fields, scientists can tackle the multifaceted challenges posed by these conditions more effectively. Initiatives that encourage interdisciplinary collaboration, such as those seen in Stevens’ research, foster innovative solutions that might not have emerged in isolated studies.
Beth Stevens’ partnerships with other researchers and institutions exemplify the power of collaboration. By engaging with geneticists, neurologists, and other specialists, her work on microglia and synaptic pruning has the potential to reach new heights. This collaborative approach not only enhances the depth of research but also accelerates the translation of findings into meaningful therapies for Alzheimer’s patients, illustrating how teamwork is crucial in advancing the field.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are vital components of the brain’s immune system and play a significant role in Alzheimer’s disease research. These cells are responsible for patrolling the brain for damage and removing dead cells, a process known as synaptic pruning. In the context of Alzheimer’s, improper microglial activity can lead to excessive pruning and contribute to the progression of the disease, as highlighted in the research led by Beth Stevens.
How does microglial research relate to neurodegenerative diseases?
Microglial research is crucial in understanding neurodegenerative diseases like Alzheimer’s and Huntington’s disease. These immune cells not only protect the brain by clearing damaged cells but also participate in synaptic pruning during development. Abnormal pruning carried out by microglia can exacerbate neurodegenerative conditions, making this research essential for developing potential treatments.
What are the implications of Beth Stevens’ research on microglia in Alzheimer’s disease?
Beth Stevens’ research on microglia reveals their dual role in protecting the brain and potentially exacerbating Alzheimer’s disease through improper synaptic pruning. Her findings have led to the identification of new biomarkers and therapeutic targets for neurodegenerative diseases, representing a pivotal advancement in Alzheimer’s disease research and treatment possibilities.
What insights does microglial research provide about the brain’s immune system?
Microglial research offers profound insights into the brain’s immune system by illustrating how these cells respond to injury and disease. These insights are critical for understanding how inflammation and immune responses may influence the development of neurodegenerative diseases such as Alzheimer’s, highlighting the importance of microglia in maintaining brain health.
How do microglial cells affect synaptic pruning during brain development?
Microglial cells play a key role in synaptic pruning, a natural process during brain development where excess synapses are eliminated to enhance neural efficiency. However, improper regulation of this pruning can lead to issues associated with Alzheimer’s disease and other neurodegenerative disorders, as demonstrated by the research from Beth Stevens that emphasizes the complexities of microglial function.
Why is foundational research important in the field of microglial studies?
Foundational research is crucial in microglial studies as it lays the groundwork for understanding complex brain functions and diseases. Beth Stevens emphasizes that such curiosity-driven investigations are essential for uncovering mechanisms that govern microglial behavior, which can ultimately inform the development of new strategies to combat Alzheimer’s disease and related disorders.
| Key Point | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, removing damaged cells and pruning synapses. |
| Impact on Alzheimer’s Disease | Improper pruning by microglia can lead to neurodegenerative diseases like Alzheimer’s and Huntington’s. |
| Research Funding | Beth Stevens’ research has been significantly funded by NIH and other federal agencies. |
| Foundation of Basic Science | Curiosity-driven research has led to discoveries fundamental to understanding microglial functions. |
| Recognition | Stevens received a MacArthur grant for her significant contributions to understanding microglia. |
| Future Implications | Research has potential to develop new biomarkers and treatments for Alzheimer’s and other disorders. |
Summary
Microglial research is crucial in the understanding of neurodegenerative diseases such as Alzheimer’s, as it uncovers the roles and functions of these immune cells in the brain. Beth Stevens’ groundbreaking work demonstrates how the improper functioning of microglia affects brain health and contributes to disease progression. Her findings highlight the importance of continued support for basic science research, which can ultimately lead to innovative treatments and a better future for those affected by Alzheimer’s. This ongoing investigation not only enhances our understanding of the brain but also paves the way for advancements that could significantly improve patient care.

Alzheimer’s Disease Research: Insights from Beth Stevens
Alzheimer’s disease research is at the forefront of scientific exploration, aiming to unravel the complex mechanisms behind this devastating condition. Under the leadership of pioneering neuroscientist Beth Stevens, significant advancements have been made in understanding the role of microglial cells, which act as the brain’s immune defenders. These cells are crucial for maintaining neuronal health by removing debris and refining synaptic connections, yet their malfunction can contribute to neurodegenerative diseases like Alzheimer’s. Findings from the Stevens Lab at Boston Children’s Hospital highlight how abnormal microglial activity can lead to the progression of such disorders, paving the way for innovative Alzheimer’s treatment strategies. As the aging population in the United States continues to grow, the urgency for effective therapies has never been more critical.
In the realm of neurodegenerative disease exploration, advances in Alzheimer’s disease research are crucial for understanding and combating cognitive decline. Innovators like Beth Stevens are reshaping our knowledge of the brain’s immune response through the study of microglial cells, which are vital for neural maintenance and repair. These immune cells help protect the brain by eliminating waste and optimizing neural connections, yet dysregulation in their function can exacerbate conditions like Alzheimer’s. The insight generated from studies at institutions like Boston Children’s Hospital is not only essential for early detection but also for developing impactful treatments for millions affected by these conditions. As research delves deeper into immune mechanisms and their implications for brain health, the prospects for combating Alzheimer’s continue to evolve.
Understanding the Role of Microglial Cells in Alzheimer’s Disease
Microglial cells are integral to brain health and play a crucial role in the progression of Alzheimer’s disease. These immune cells are responsible for maintaining homeostasis in the central nervous system (CNS) by continuously monitoring the brain environment. Their primary functions include clearing out dead neurons, managing inflammation, and supportively reshaping synapses. Recent studies have indicated that dysfunctional microglial activity can contribute to the pathogenesis of neurodegenerative diseases such as Alzheimer’s, where excessive synaptic pruning may lead to cognitive deficits. Thus, understanding how microglial cells operate can unveil potential therapeutic targets for Alzheimer’s treatment.
Research led by Beth Stevens at Boston Children’s Hospital has revealed that microglial cells do not merely act as passive scavengers; instead, they actively participate in the brain’s development and response to injury. By studying these cells, scientists have started to identify biomarkers that could signal the onset of Alzheimer’s disease even before symptoms manifest. This discovery is pivotal because early detection can significantly change the management of Alzheimer’s, leading to timely interventions and potentially slowing the disease’s progression. The ongoing exploration of microglial function is reshaping our understanding of neurodegenerative disease pathology.
Beth Stevens: Pioneering Research in Neurodegenerative Diseases
Beth Stevens has emerged as a leading figure in the field of neuroscience, particularly in her innovative research on microglial cells and their connection to neurodegenerative diseases. Her dedication to uncovering the complexities of the brain’s immune system has been instrumental in changing the landscape of Alzheimer’s research. Through her efforts at the Stevens Lab, she has illuminated how improper microglial pruning processes can exacerbate conditions like Alzheimer’s and Huntington’s diseases. By focusing on these immune cells, Stevens is paving the way for novel therapies that target the underlying mechanisms of neurodegeneration.
Stevens’ groundbreaking work is not only crucial for basic science but also has profound implications for public health as the incidence of Alzheimer’s continues to rise. With predictions suggesting a doubling of cases by 2050, Stevens’ research could lead to earlier diagnosis and more effective treatments, relieving the estimated financial burden associated with caregiving. This highlights the importance of continued funding and support for scientific discoveries that drive innovation in Alzheimer’s treatment and ultimately improve the quality of life for millions affected by the disease.
The Impact of Federal Funding on Alzheimer’s Research
Federal funding plays a critical role in advancing research on Alzheimer’s and other neurodegenerative diseases. For researchers like Beth Stevens, support from institutions like the National Institutes of Health (NIH) has been vital for fostering innovative ideas. These resources provide the necessary tools and platforms for scientists to investigate intricate biological systems, including the functions of microglial cells in neurodegeneration. Without this backing, many fundamental studies could remain unexplored, stalling progress in understanding diseases like Alzheimer’s.
The strategic allocation of federal funds directs focus on high-impact areas within Alzheimer’s research, facilitating developments in novel therapies and biomarkers. As the aging population continues to grow, the urgency for effective Alzheimer’s treatment escalates. This funding not only nurtures existing research but also motivates upcoming scientists to pursue careers in neuroscience, ensuring that momentum in Alzheimer’s research continues to build. Investing in research today promises significant returns in health outcomes and economic savings tomorrow.
Neuroscience Breakthroughs: A Path to Alzheimer’s Treatment
Neuroscience breakthroughs are continuously reshaping the pathways we understand Alzheimer’s disease and its treatment options. Through ongoing studies, researchers are identifying how various cellular mechanisms, particularly involving microglial cells, contribute to neurodegeneration. Beth Stevens’ research has illuminated how selective synaptic pruning can lead to loss of neuronal connections, impacting cognition. By making sense of these complex interactions, scientists can explore innovative treatment strategies that may enhance brain health and restore lost functions associated with Alzheimer’s.
Furthermore, with an emphasis on the relationship between genetic factors and neurodegenerative diseases, the field is witnessing a wave of exciting developments. The identification of new biomarkers associated with microglial function opens avenues for early detection, allowing healthcare providers to act preemptively. As this field evolves, there is potential for personalized treatment regimes that target specific pathways involved in Alzheimer’s, increasing the effectiveness of therapeutic interventions and providing hope to millions affected by cognitive decline.
Boston Children’s Hospital: Hub of Alzheimer’s Innovations
Boston Children’s Hospital has positioned itself as a leading institution in the fight against Alzheimer’s, hosting pioneering researchers such as Beth Stevens. The collaboration between the hospital and the Broad Institute of MIT and Harvard primes the environment for groundbreaking discoveries related to neurodegenerative diseases. As a center that emphasizes a multidisciplinary approach, it attracts diverse talents in neuroscience and immunology, fostering an ecosystem where innovative ideas can flourish and lead to significant advancements in Alzheimer’s treatment.
The research conducted within the walls of Boston Children’s Hospital does not just focus on symptom management but seeks to unravel the very mechanisms behind diseases like Alzheimer’s. By integrating basic science with clinical applications, researchers aim for tangible outcomes that transcend laboratory findings. As the knowledge generated here feeds into broader medical practices, it offers hope for improved diagnostic tools and more effective therapies, benefiting patients and families affected by Alzheimer’s disease.
Innovative Therapies Emerging from Alzheimer’s Research
The relentless pursuit of innovative therapies has become a hallmark of Alzheimer’s research. Thanks to pioneering scientists like Beth Stevens, new treatment avenues focused on modulating microglial cells are being explored. Understanding how these cells can be harnessed to optimize synaptic pruning opens the door for the development of drugs that may prevent or reverse cognitive decline associated with Alzheimer’s. Harnessing the immune system’s capabilities offers promising therapeutic strategies that could fundamentally alter the course of the disease.
Innovation in the pharmaceutical realm is also inspired by the fundamental neuroscience studies that elucidate the complexities of Alzheimer’s. Collaborative efforts within academic institutions and industry are yielding novel compounds aimed at enhancing microglial function, thereby improving neuronal health. As this area of research matures, we may witness a shift in the standard care model for Alzheimer’s, bringing forth treatments that target the disease at its source and enhancing the lives of those impacted.
The Future of Alzheimer’s Care: Lessons from Research
The future of Alzheimer’s care lies firmly rooted in the lessons learned from ongoing research efforts. As Beth Stevens and her colleagues delve deeper into the mechanics of microglial involvement, the insights gained are poised to reshape our understanding of disease management. A comprehensive approach that combines basic science with patient care will inform future clinical practices. This alignment ensures that treatment strategies evolve such that they are informed by the latest discoveries in neuroscience.
Moreover, improving the quality of care for Alzheimer’s patients will necessitate a paradigm shift in how we approach neurodegenerative diseases. The advancements in research underscore the importance of not only developing medications but also increasing public awareness and understanding of Alzheimer’s. Initiatives focused on education, early detection, and community support will complement medical advancements, ultimately enhancing the overall efficacy of care and preserving dignity for those navigating the complexities of Alzheimer’s.
Microglial Cells: The New Frontier in Alzheimer’s Research
Microglial cells have emerged as a new frontier in Alzheimer’s research, capturing the attention of scientists worldwide. Beth Stevens’ groundbreaking investigations into these cells reveal their pivotal role not just in maintaining brain health but also in the progression of Alzheimer’s disease. As the brain’s immune responders, microglial cells interact with neurons to ensure cerebral homeostasis, but when dysfunction arises, they may inadvertently contribute to neurodegeneration through excessive synaptic pruning. Understanding these processes is vital for designing targeted interventions that leverage microglial capabilities in therapeutic frameworks.
The potential implications of microglial cell research are profound, as they may not only lead to advancements in Alzheimer’s treatment but also offer insights relevant to other neurodegenerative diseases. By elucidating the pathways that govern microglial behavior, researchers can pave the way for innovative strategies that restore balance in the brain’s microenvironment. Thus, the pursuit of knowledge surrounding microglial cells stands at the forefront of efforts to combat Alzheimer’s disease and improve the lives of millions worldwide.
Collaboration in Alzheimer’s Research: A Necessity for Progress
Collaboration in Alzheimer’s research is paramount for driving progress, especially in the complexities surrounding neurodegenerative diseases. Institutions like Boston Children’s Hospital foster environments where multidisciplinary teams can converge to share insights and tackle the challenges posed by Alzheimer’s. Collaborative efforts between neuroscientists, clinicians, and biochemists enable the integration of diverse perspectives, which is essential for uncovering comprehensive solutions to the pressing issues of disease management and treatment.
The synergy created through such partnerships enhances the potential for breakthroughs, as researchers can pool resources and expertise to explore groundbreaking concepts in Alzheimer’s treatment. With significant progress already achieved by teams studying microglial cells under the leadership of scientists like Beth Stevens, collaboration serves as a catalyst for new discoveries that have the power to transform the landscape of Alzheimer’s care. This interconnected approach aligns science closely with patient needs, ultimately leading to better health outcomes.
Frequently Asked Questions
What is the significance of Beth Stevens’ research on microglial cells in Alzheimer’s disease research?
Beth Stevens’ research on microglial cells is pivotal in Alzheimer’s disease research as it reveals how these brain immune cells prune synapses. Aberrant pruning by microglia can lead to neurodegenerative diseases, including Alzheimer’s, by impairing synaptic health. Her findings contribute to developing new treatments and biomarkers, which are crucial for early detection of Alzheimer’s disease.
How do microglial cells contribute to the understanding of neurodegenerative diseases like Alzheimer’s?
Microglial cells play a critical role in neurodegenerative diseases such as Alzheimer’s by maintaining brain health through synaptic pruning. However, when this process goes awry, it can contribute to disease progression. Research led by Beth Stevens at Boston Children’s Hospital highlights the importance of understanding microglial function in order to develop effective therapies and intervention strategies for Alzheimer’s disease.
What role does Boston Children’s Hospital play in advancing Alzheimer’s treatment research?
Boston Children’s Hospital is at the forefront of Alzheimer’s treatment research, particularly through the work of scientists like Beth Stevens. The hospital’s partnership with the Broad Institute and its focus on microglial cells have facilitated groundbreaking discoveries that enhance understanding of Alzheimer’s disease and promote the development of new, targeted treatments for patients.
What are the implications of Stevens’ findings for Alzheimer’s disease treatment in the future?
Stevens’ findings suggest that targeting microglial function could lead to innovative treatments for Alzheimer’s disease. By understanding how microglial cells participate in synapse pruning and their overall role in brain health, researchers can develop new therapeutic strategies aimed at improving cognitive function and slowing disease progression among the millions affected by Alzheimer’s.
Why is basic science crucial for breakthroughs in Alzheimer’s disease research?
Basic science is vital for breakthroughs in Alzheimer’s disease research because it lays the foundational knowledge necessary to understand complex biological processes. Researchers like Beth Stevens emphasize that exploring fundamental mechanisms, such as microglial activity, can lead to unexpected discoveries that ultimately translate into effective treatments for Alzheimer’s and other neurodegenerative diseases.
| Key Point | Details |
|---|---|
| Research Significance | Explores the role of microglial cells in the brain’s immune response, crucial for understanding Alzheimer’s. |
| Microglial Cells Role | They clear damaged cells and prune synapses, impacting neurodegenerative diseases like Alzheimer’s. |
| Transformative Findings | Aberrant pruning of synapses potentially contributes to Alzheimer’s and similar disorders. |
| Future of Treatment | Stevens’ research lays the groundwork for new medicines and early detection biomarkers. |
| Increasing Impact | With millions affected by Alzheimer’s, Stevens’ findings could significantly impact treatment costs and management. |
Summary
Alzheimer’s disease research is at a pivotal point, driven by groundbreaking discoveries by scientists like Beth Stevens. Her exploration of microglial cells highlights how crucial these immune cells are in the context of neurodegenerative diseases, particularly Alzheimer’s. Stevens’ work suggests that improper synaptic pruning can contribute to brain deterioration, which not only helps in understanding the disease’s mechanisms but may also lead to innovative treatments. As the population ages and the prevalence of Alzheimer’s is expected to rise dramatically, research like this is critical to developing effective interventions and improving the lives of millions affected by the disease.